
350
YEARS
OF
SCIENCE
49
© World History Archive - Alamy
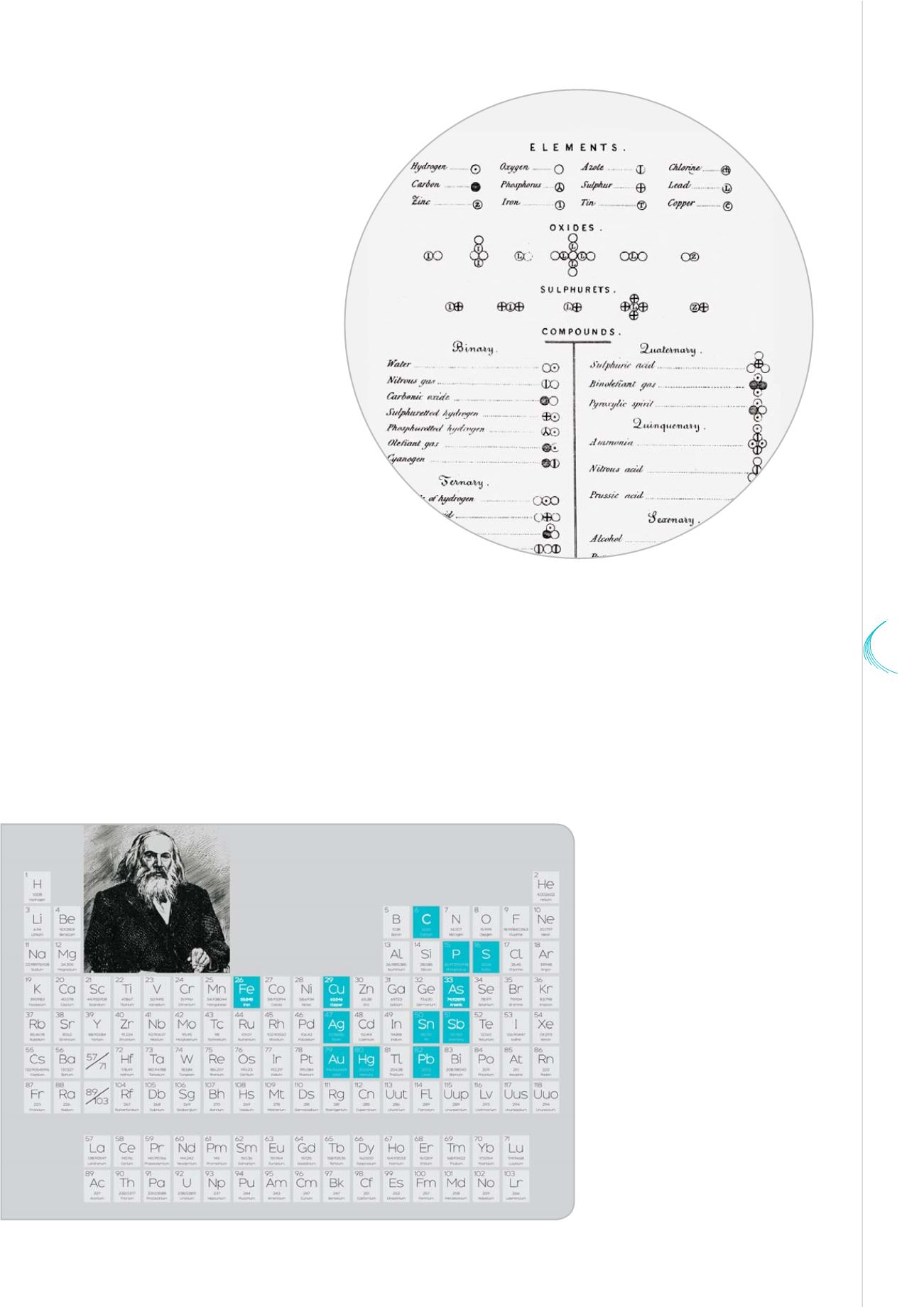
John Dalton's table of atomic symbols
The periodic table of elements, published by Dmitri Mendeleev in 1869, illustrates the
essential contributions analytical chemistry made in the 18
th
century. However, at that
time, only a dozen chemical elements (in blue) were known.
© Paul Stringer - Juulijs - Fotolia
John Dalton confirmed Democritus’ model,
drawing a distinction between the
atom and the molecule. In his New
System of Chemical Philosophy,
published in 1808, he showed
that air was made of a mixture
of four gases: nitrogen,
oxygen, carbon dioxide and
water vapour! It was the
birth of the modern atomic
theory.
From analysis
to synthesis
The concept of atoms as the
elementary compounds of matter was
accepted, but it still remained to know what
an atom was. In the early 18
th
century, only a dozen
elements had been identified, such as gold, silver, mercury,
lead, copper, sulphur and carbon. There still remained a hundred other elements
to be found in order to know at last what matter was made of. Analysis thus became the chemists’ first
task: they discovered chromium and beryllium (Vauquelin), boron (Thénard), bromine (Balard) and silicon
(Berzelius). About sixty chemicals were thus discovered over the following two centuries. They were listed
in the periodic table published by the Russian chemist Mendeleev in 1869. This classification reveals how
clever and sharp-eyed the
chemists of the 19
th
century
were. At the time, electrons
and the structure of the atoms
were still unknown, the latter
being only described in 1913
by Niels Bohr. Each element
was only defined by its mass
and properties. Moreover,
many elements were still to
be discovered. It was thus
necessary to leave blank
spaces, which would, by
some miracle, coincide with
the elements that would be


















